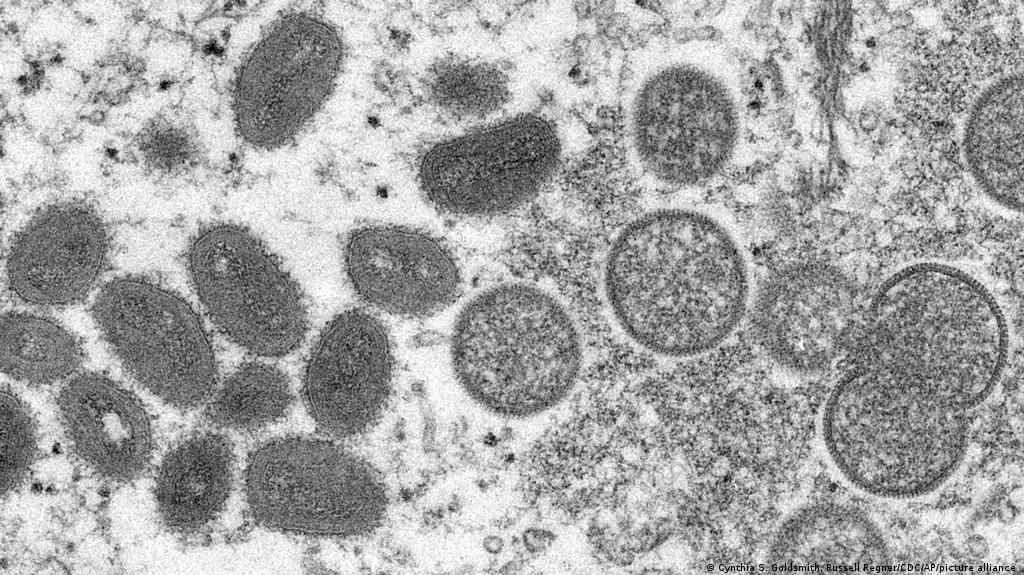
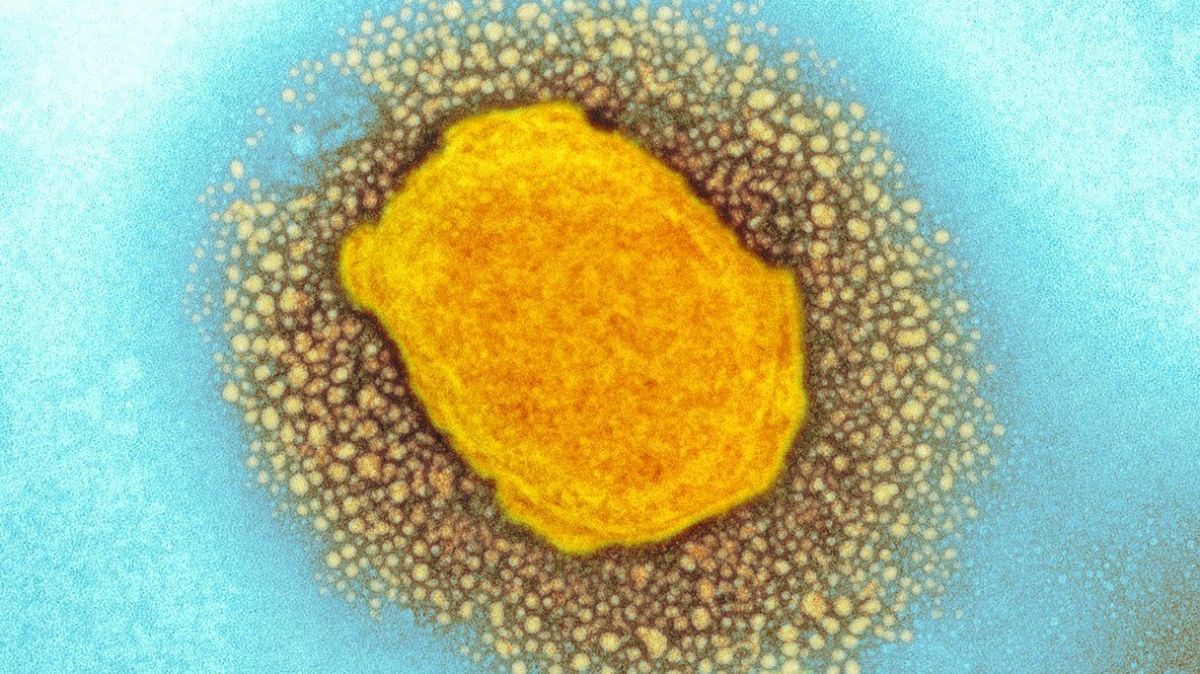
Post-exposure vaccination started on July 16 in Portugal,
with a total of 437 vaccinated until September 12, 2022, with the DGS
discussing and reviewing the standard “Approach to cases of human infection
with Monkeypox virus”, framework for the administration of reduced doses,
according to new guidelines from the European Medicines Agency (EMA).
In the standard the DGS states that “the conditions for
operationalization/availability and equity in the management of the limited
stock of vaccines for the approach to preventive vaccination and the respective
definition of eligibility criteria are also being updated”, in addition to post-exposure
vaccination.
The EMA considers that the vaccine authorised in the
European Union against Monkeypox can also be administered as an intradermal
injection at a lower dose, allowing the existing doses to be multiplied by five
times.
Until now, the vaccine has only been administered to people
who have had risky contacts and the objective is to preventively vaccinate
other groups that will be defined by the DGS and that may include sex workers,
people who undergo PREP - Pre-Exposure Prophylaxis to HIV and health
professionals.
With regard to the clinical approach of pregnant women
confirmed with Monkeypox infection, the DGS states that they must be followed
up in a high-risk obstetrics consultation at a differentiated perinatal support
hospital, which involves specific procedures for pregnancy surveillance and
fetal monitoring.